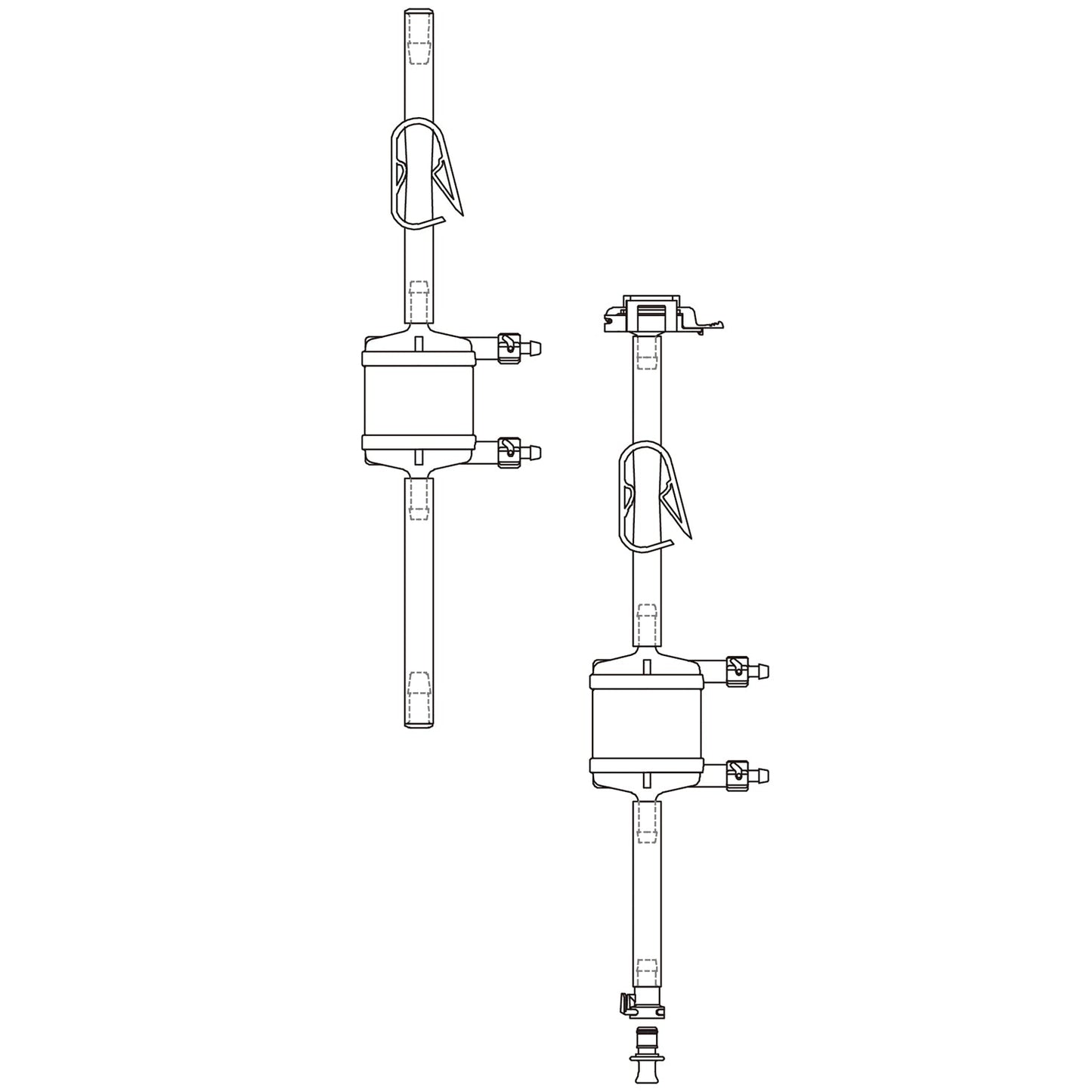
Lifecube™ SA Single-Use Filter Sets with STF TPE Tubing and Plugs, 1 set/pk
Couldn't load pickup availability
Cobetter Lifecube™ single-use tubing assemblies are used for interconnection of different unit operations in a wide range of applications. With our tubing assemblies, you can easily connect multiple containers, vessels and process lines in one system, minimizing the risk of cross-contamination, saving time and reducing costs.
The Lifemeta™ STF high-performance tubing is extruded from medical-grade thermoplastic elastomer (TPE) material. Compatible with common tube sealers and aseptic tube welders, achieving excellent performance of frequent aseptic disconnection and connection of tubings.
The Lifecube™ single-use filter sets consist of a 0.2 micron validated sterilizing-grade Bricap C/L capsule filter. The incorporated Purcise SLE polyethersulfone (PES) membrane demonstrates very low protein binding and 1-14 pH range.
Specifications
| Connections | Port 1 | Tubing Plug | ||||
| Port 2 | Tubing Plug | |||||
| Dimensions | Tube Length | 50/100/250 cm | ||||
| Tubing Size | 1/8" ID x 1/4" OD, 1/4" ID x 7/16" OD, 3/8" ID x 5/8" OD, 1/2" ID x 3/4" OD |
|||||
| Physical Properties | Tubing Material | Lifemeta™ STF TPE Tubing Lifemeta™ STT Pt-cured Silicone Tubing |
||||
| Filter Material | Purcise™ SLE polyethersulfone (PES) membrane | |||||
| Pore size | 0.2μm (with 0.45μm prefilter) | |||||
| Filter Type |
|
|||||
| EFA |
|
|||||
| Sterility | Delivery Condition | Sterile | ||||
| Sterilization Method | Gamma Irradiation | |||||
| Package | Pack Size | 1 set |
Features
- Common connectors and tubing assembly available
- Double-layer packaging, sterilized by 25~45 kGy gamma irradiation
- Non-sterile packaging, autoclavable
- Flexible customized solutions
Applications
- Fluid transfer between Single-use and stainless steel system
- Fluid Transfer between different types of connectors
- Upstream and downstream system switching
- PUPSIT system (Pre-Use and Post-Sterilization Integrity Test)
- Connect and transfer of Single-use products from different brands
- Temporary feeding and filtration, etc
Regulatory Compliance
- ISO 9001:2015 quality management system
- ISO Class 4.8 / ISO 7 cleanroom production
- 100% leak testing
- ADCF raw materials, complies with FDA 21 CFR Part 177-182(Indirect food additives)
- Meet the requirement of current USP <87> Class VI, Biological Reactivity Test, In Vitro
- Meet the criteria of current USP <88> Class VI, Biological Reactivity Test, In Vivo
- Aqueous extraction contains <0.25 EU/mL as determined by Limulus Amebocyte Lysate (LAL), USP <85>
- Particulate matter in the product eluent meets the requirements in USP <788> for large volume parenterals
- Verified gamma irradiation dose according to ISO® 11137
- Cobetter_Lifecube_SA_Single-Use_Assemblies_Catalog_250710 Download