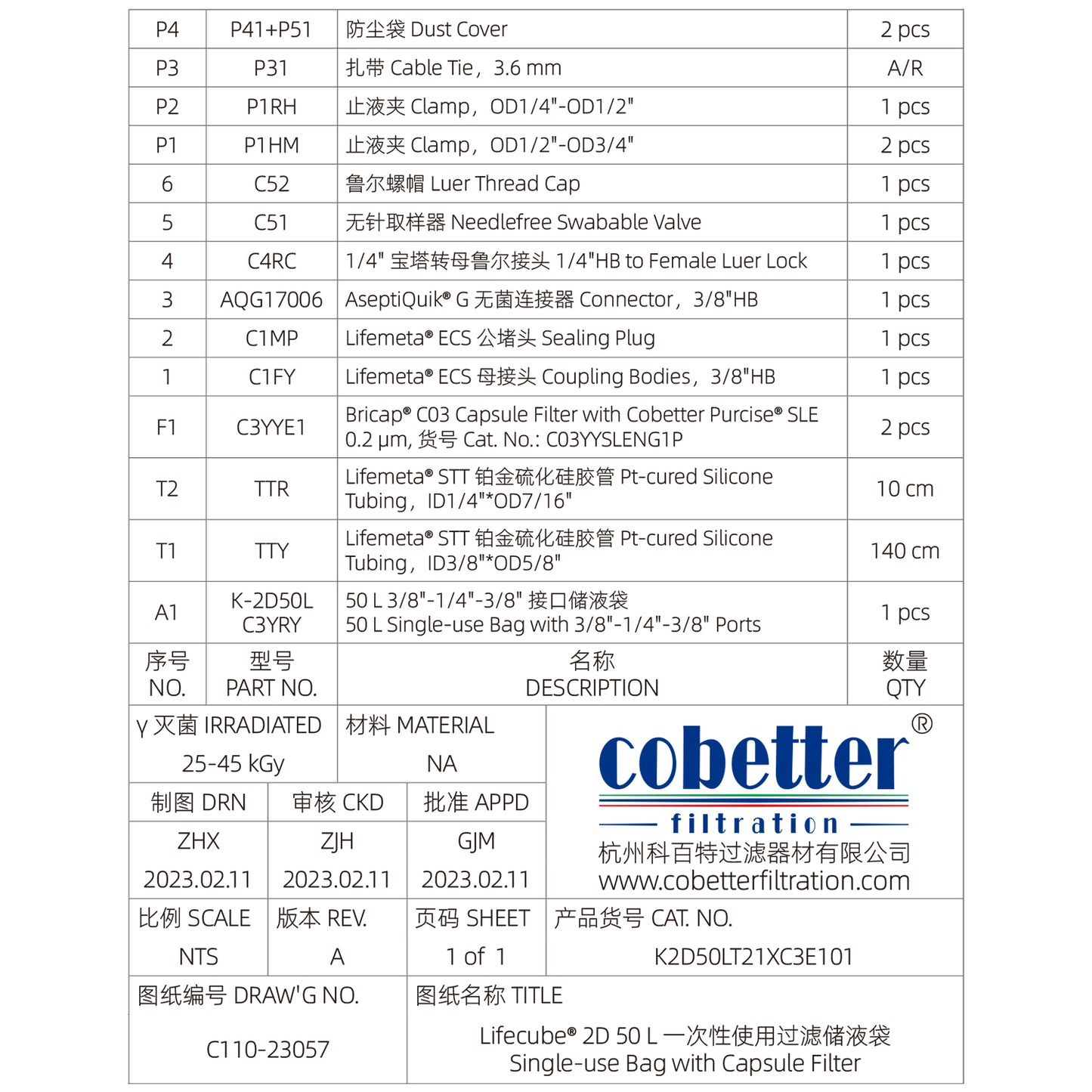
Standard Single-Use 2D Bag Assemblies with Capsule Filter, Sterile and Ready-to-use
Couldn't load pickup availability
Lifecube™ single-use 2D bag assemblies with filter are sterile, pre-assembled systems designed for fluid handling in biopharmaceutical and life sciences applications. The single-use bag assemblies integrate a filtration unit, ensuring aseptic processing while eliminating cross-contamination risks.
Bag Assemblies with Topene™ Film
Cobetter Topene RB blown film designed for biological processes is composed of ULDPE (ultra-low density polyethylene), EVA (ethylene-acetic acid ethylene copolymer), EVOH (ethylene-vinyl alcohol copolymer) and LDPE (low density polyethylene). Cobetter Lifecube™ 2D bioprocess bags are available in 1, 2, 5, 10, 20, 35 and 50 L sizes.
Tubing
Available in both Pt-cured Silicone and TPE (thermoplastic elastomer) tubing.
Inlet and outlet Connectors
Multiple types and sizes of connectors are available including ECS Connector, Aseptic Connector, TC, and Plug.
Sampling Port
Needlefree swabable valve, sampling plug, male and female Lure Lock.
Capsule Filter
Cobetter Bricap™ C / L / T series sterilizing-grade filters.
Cobetter Bag Assemblies Advantages:
- Reduced Contamination Risk: Pre-sterilized and ready-to-use, eliminating cleaning validation
- Cost-Efficient: Lower cost (no CIP/SIP systems) and reduced downtime
- Scalability: from R&D to commercial production
Regulatory Compliance: Meets cGMP, USP Class VI, and FDA standards. - Flexibility: Compatible with single-use bioreactors and downstream equipment.
Specifications
| Configuration | Standard 1 | Standard 2 |
| Single-Use Bag | 5L 2D Bag with 1/4"-1/4"-1/4" Ports | 50L 2D Bag with 3/8"-1/4"-3/8" Ports |
| Tubing | STT Platinum Cured Silicone Tubing, 1/4" ID | STT Platinum Cured Silicone Tubing, 3/8" ID |
| Capsule Filter | Bricap™ C02 with 0.2μm PES, Filtration Area: 420cm² | Bricap™ C03 with 0.2μm PES, Filtration Area: 660cm² |
| Sterility | Sterile by Gamma Irradiation | |
Regulatory Compliance
- ISO 9001:2015 quality management system
- ISO Class 4.8 / ISO 7 cleanroom production
- ADCF raw materials, complies with FDA 21 CFR Part 177-182(Indirect food additives)
- Meet the requirement of current USP <87> Class VI, Biological Reactivity Test, In Vitro
- Meet the criteria of current USP <88> Class VI, Biological Reactivity Test, In Vivo
- Aqueous extraction contains <0.25 EU/mL as determined by Limulus Amebocyte Lysate (LAL), USP <85>
- Particulate matter in the product eluent meets the requirements in USP <788> for large volume parenterals
- Verified gamma irradiation dose according to ISO® 11137