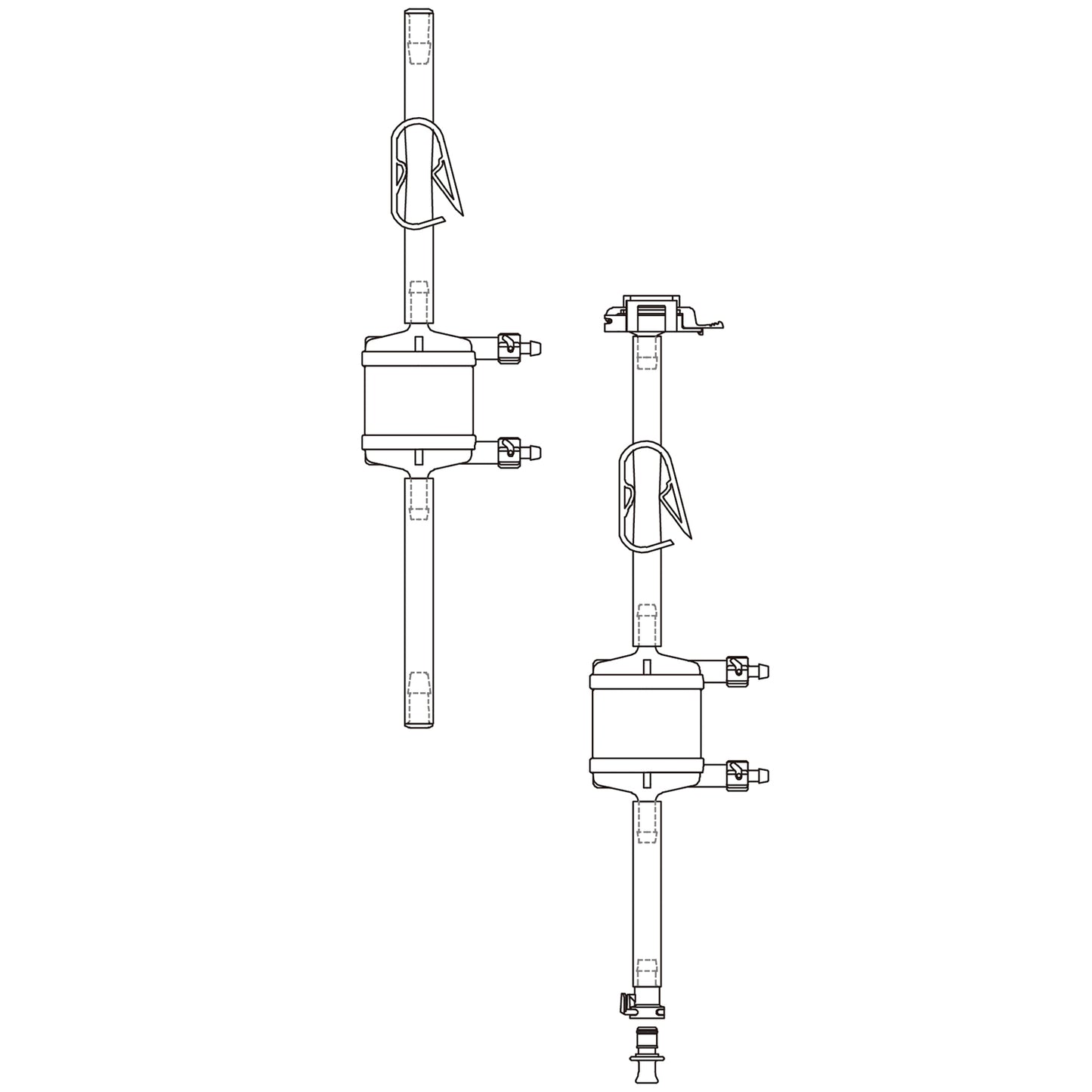
Lifecube™ SA Single-Use Filter Sets with C-Flex® Tubing and Plugs, 1 set/pk
Couldn't load pickup availability
Cobetter Lifecube™ single-use tubing assemblies are used for interconnection of different unit operations in a wide range of applications. With our tubing assemblies, you can easily connect multiple containers, vessels and process lines in one system, minimizing the risk of cross-contamination, saving time and reducing costs.
C-Flex® is the original patented thermoplastic elastomer tubing specifically designed to meet the critical demands of pharmaceutical and biopharmaceutical applications for fluid processing with excellent heat seal and sterile weld capabilities.
The Lifecube™ single-use filter sets consist of a 0.2 micron validated sterilizing-grade Bricap C/L capsule filter. The incorporated Purcise SLE polyethersulfone (PES) membrane demonstrates very low protein binding and 1-14 pH range.
Specifications
| Connections | Port 1 | Tubing Plug | ||||
| Port 2 | Tubing Plug | |||||
| Dimensions | Tube Length | 50/100/250 cm | ||||
| Tubing Size | 1/8" ID x 1/4" OD, 1/4" ID x 7/16" OD, 3/8" ID x 5/8" OD, 1/2" ID x 3/4" OD |
|||||
| Physical Properties | Tubing Material | C-Flex® 374 TPE | ||||
| Filter Material | Purcise™ SLE polyethersulfone (PES) membrane | |||||
| Pore size | 0.2μm (with 0.45μm prefilter) | |||||
| Filter Type |
|
|||||
| EFA |
|
|||||
| Sterility | Delivery Condition | Sterile | ||||
| Sterilization Method | Gamma Irradiation | |||||
| Package | Pack Size | 1 set |
Features
- Common connectors and tubing assembly available
- Double-layer packaging, sterilized by 25~45 kGy gamma irradiation
- Non-sterile packaging, autoclavable
- Flexible customized solutions
Applications
- Fluid transfer between Single-use and stainless steel system
- Fluid Transfer between different types of connectors
- Upstream and downstream system switching
- PUPSIT system (Pre-Use and Post-Sterilization Integrity Test)
- Connect and transfer of Single-use products from different brands
- Temporary feeding and filtration, etc
Regulatory Compliance
- ISO 9001:2015 quality management system
- ISO Class 4.8 / ISO 7 cleanroom production
- 100% leak testing
- ADCF raw materials, complies with FDA 21 CFR Part 177-182(Indirect food additives)
- Meet the requirement of current USP <87> Class VI, Biological Reactivity Test, In Vitro
- Meet the criteria of current USP <88> Class VI, Biological Reactivity Test, In Vivo
- Aqueous extraction contains <0.25 EU/mL as determined by Limulus Amebocyte Lysate (LAL), USP <85>
- Particulate matter in the product eluent meets the requirements in USP <788> for large volume parenterals
- Verified gamma irradiation dose according to ISO® 11137
- Cobetter_Lifecube_SA_Single-Use_Assemblies_Catalog_250710 Download