Lifecube™ MS Single-use Mixing Bag Sterile by Gamma Irradiation
Couldn't load pickup availability
Cobetter Lifecube Single-use Mixing Bags offer no-clean, many specifications, pre-sterilization, easy to install and flexibility of use, etc. The Lifecube Single-use Mixing bags are made of multi-layer co-extrusion film, feature good physical and chemical properties and extremely low gas permeability. Lifecube Single-use Mixing bags are suitable for a wide range of mixing steps in upstream and downstream processes.
Specifications
| Materials | Mixing Type |
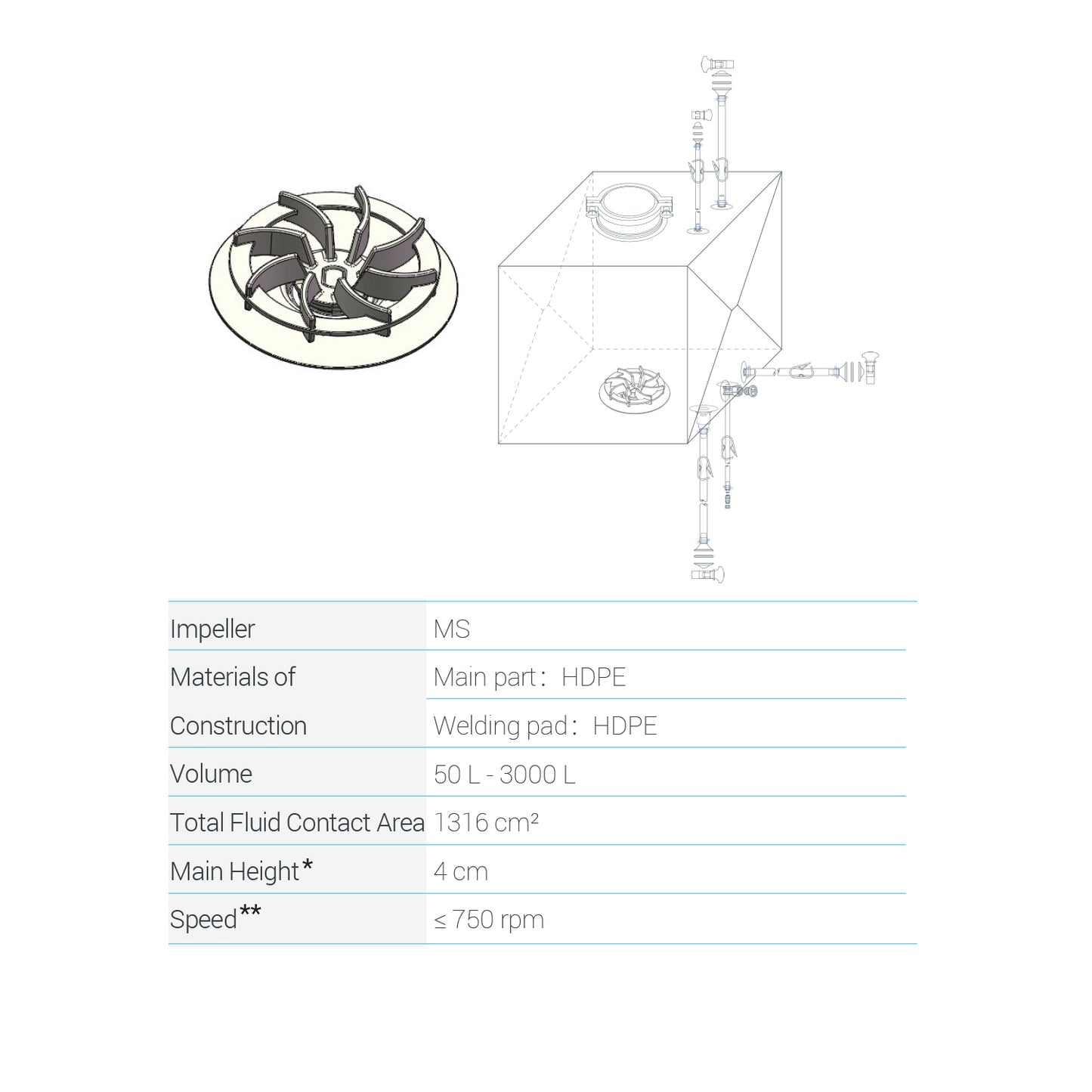
Cobetter Lifecube MS Single-use Mixing Bags |
| Material | Cobetter Topene RB Film | |
|
Impeller (MS) |
Materials of Construction |
Main part/ Welding pad: HDPE |
| Volume | 50L - 3000L | |
| Total Fluid Contact Area | 1316 cm² | |
| Main Height* | 4 cm | |
| Speed** | ≤ 750 rpm | |
| Tubing and Port | Inlet |
50/100/200 L: 150cm 3/8" ID x 5/8" OD Lifemeta™ STT Pt-cured Silicone tubing + ECS Female Quick Connector with plug; 400~3000 L: 150cm 1/2" ID x 3/4" OD Lifemeta™ STT Pt-cured Silicone tubing + ECM Female Quick Connector with plug. |
| Outlet |
50/100/200 L: 150cm 3/8" ID x 5/8" OD Lifemeta™ STT Pt-cured Silicone tubing + ECS Male Quick Connector with plug; 400~1000 L: 150cm 1/2" ID x 3/4" OD Lifemeta™ STT Pt-cured Silicone tubing + ECM Male Quick Connector with plug; 1500~3000 L: 300cm 1/2" ID x 3/4" OD Lifemeta™ STT Pt-cured Silicone tubing + ECM Male Quick Connector with plug. |
|
| Sampling | 20cm 1/4" ID x 7/16" OD Lifemeta™ STT Pt-cured Silicone tubing + Needlefree Swabable Valve | |
| Powder Port | 4" TC (including silicone gasket, blind cap and tri-clamp) | |
| Sterility | Delivery Condition | Sterile |
| Sterilization Method | Gamma Irradiation | |
| Package | Pack Size | 1/pack |
Note:
*Height from the welding plate to the top of the impeller;
**For reference only. The mixing speed is different from bag specifications, stirring volumes, solutions and brand of mixers. Please confirm the mixing speed and efficiency according to the actual situation.
Features
- Magnetic suction/magnetic suspension mixing, integrated/split system, compatible with other brands
- Fast installation, well mixing run and easy setup
- Double-layer packaging, sterilized by 25~45 kGy gamma irradiation
- Many specifications, meeting a variety of preparation applications
- Customized bag sizes, flexibly match different specification of containers
- Customized Tubings, connectors and filters
- Sensors can be customized to allow on-line monitoring during the process
- Hardware with audit trail and automatic management functions available
Applications
- Buffer and culture medium preparation
- Semi-finished products preparation
- Batch mixing
Quality Assurance
- ISO 9001:2015 quality management system
- ISO Class 4.8/ ISO 7/ ISO 8 cleanroom production
- 100% leak testing
- ADCF raw materials, complies with FDA 21 CFR Part 177-182 (Indirect food addictives)
- Meet the requirement of current USP <87> Class VI, Biological Reactivity Test, In Vitro
- Meet the criteria of the current USP <88> Class VI, Biological Reactivity Test In Vitro
- Aqueous extraction contains < 0.25 EU/mL as determined by Limulus Amebocyte Lysate (LAL), USP <85>
- Particulate matter in the product eluent meets the requirements in USP <788> for large volume parenterals
- Verify gamma irradiation dose according to ISO 11137
Configuration Options

*Contact us for other configuration options.
- Cobetter_Lifecube_Single-use_System_RB_film_20241218 Download