0.2μm Hydrophobic PTFE Syringe Filters for Air Venting, Sterile or Autoclavable, 100/pk
Couldn't load pickup availability
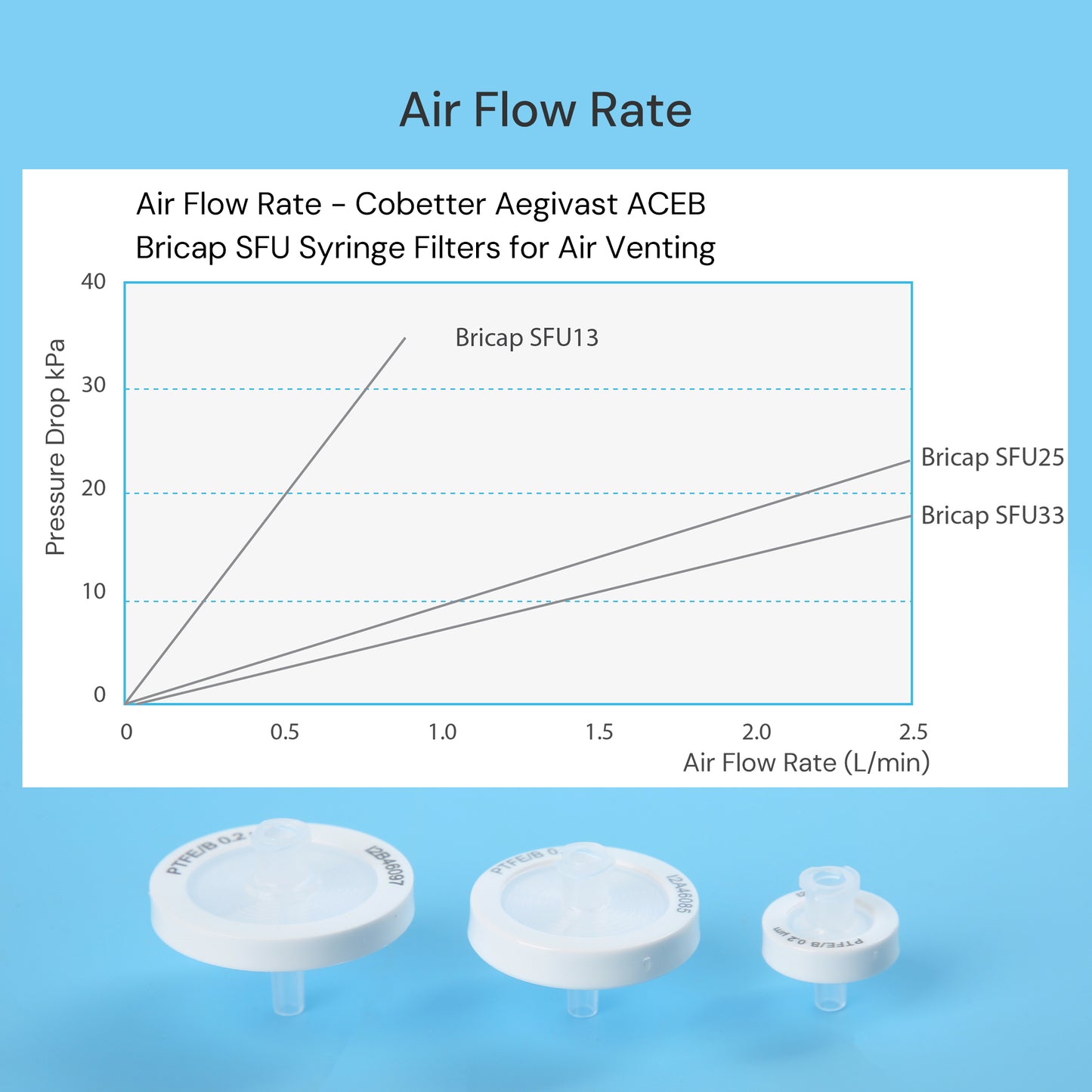
Cobetter Aegivast Vent Filter is a sterilizing-grade gas filter with high retention specifically developed for biopharmaceutical applications, available in both syringe and disc filters. The ACEB vent filter is made of a 0.2μm hydrophobic PTFE membrane composite with a polyester support layer and housed in a high-purity polypropylene casing. With a complete range of product specifications, it is highly suitable for sterile venting of small containers, bioreactors, and tubings.
Specifications
| Connections | Inlet | Female Luer Lock | |||
| Outlet | Male Luer Slip | ||||
| Physical Properties |
|
||||
| Filtration Area |
|
||||
| Maximum Operating Pressure@25°C |
|
||||
| Bubble Point@20°C, 60% IPA, 40%Water Wetted, Air Testing | ≥0.11MPa | ||||
| Materials | Housing | PP | |||
| Membrane | Hydrophobic PTFE | ||||
| Pore Size | 0.2 μm | ||||
| Sterility | Delivery Condition |
Sterile: By gamma irradiation at 25-45 kGy; Nonsterile: Can be autoclaved for 30 mins at 130 °C, 20 circles (cannot be steam sterilized in-line) |
|||
| Package | Individually packaged by paper-blister, 100pcs/pk |
Features
- Natural hydrophobic PTFE membrane
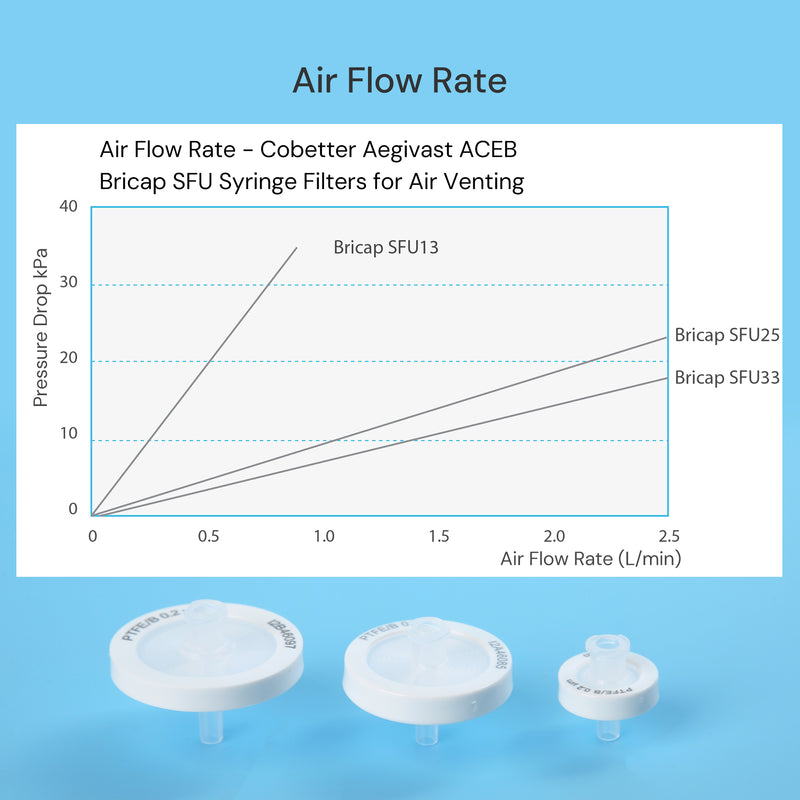
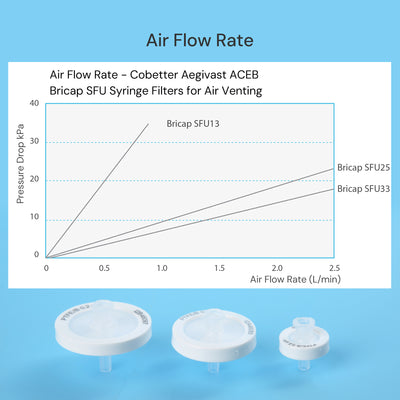
- Low pressure drop
- Reliable bacteria and fine particle retention capability
- Unique product serial number tracking system
- Can be sterilized by gamma irradiation or autoclaved
Applications
- Vent filter for fermenters, storage tanks
- Sterilization filtration of compressed air, O2, N2
- Venting filtration for single-use systems
Regulatory Compliance
- Particulate matter in injections meet the requirements in USP <788> for large volume parenterals
- Meet the criteria for a “non-fiber releasing” filter as defined in 21 CFR 210.3 (b) (6)
- Fully validated as sterilizing grade filters according to ASTM F-838 guidelines, retaining a minimum of 1 x 10⁷ CFU/cm² Brevundimonas diminuta (ATCC™ No. 19146)
- Endotoxin limit of WFI System< 0.25 EU/mL with the Limulus Amebocyte Lysate (LAL), USP <85>
- Meet the requirement of current USP <87> Class VI, Biological Reactivity Test, In Vitro
- Meet the criteria of USP <88> Class VI, Biological Reactivity Test, In Vivo
- Complies with FDA 21 CFR Part 177-182 (Indirect food additives)
- All component materials do not contain animal derived components
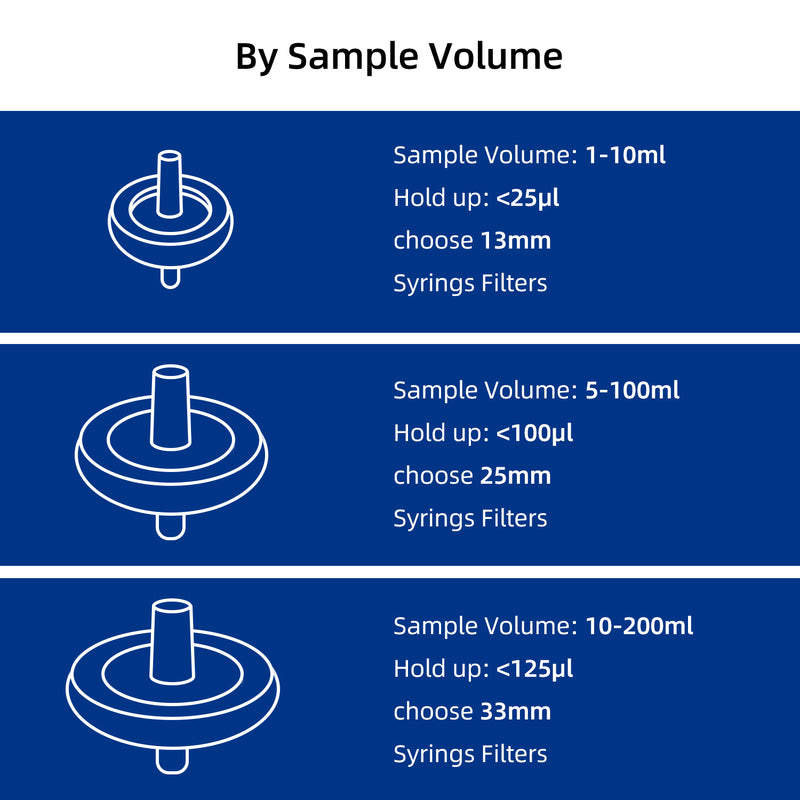
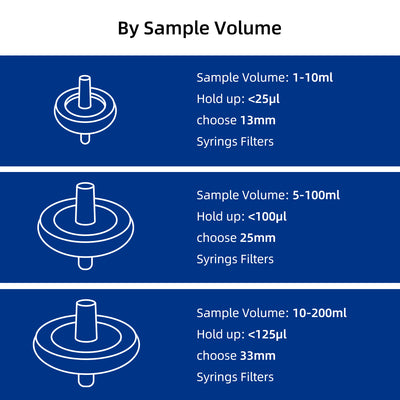
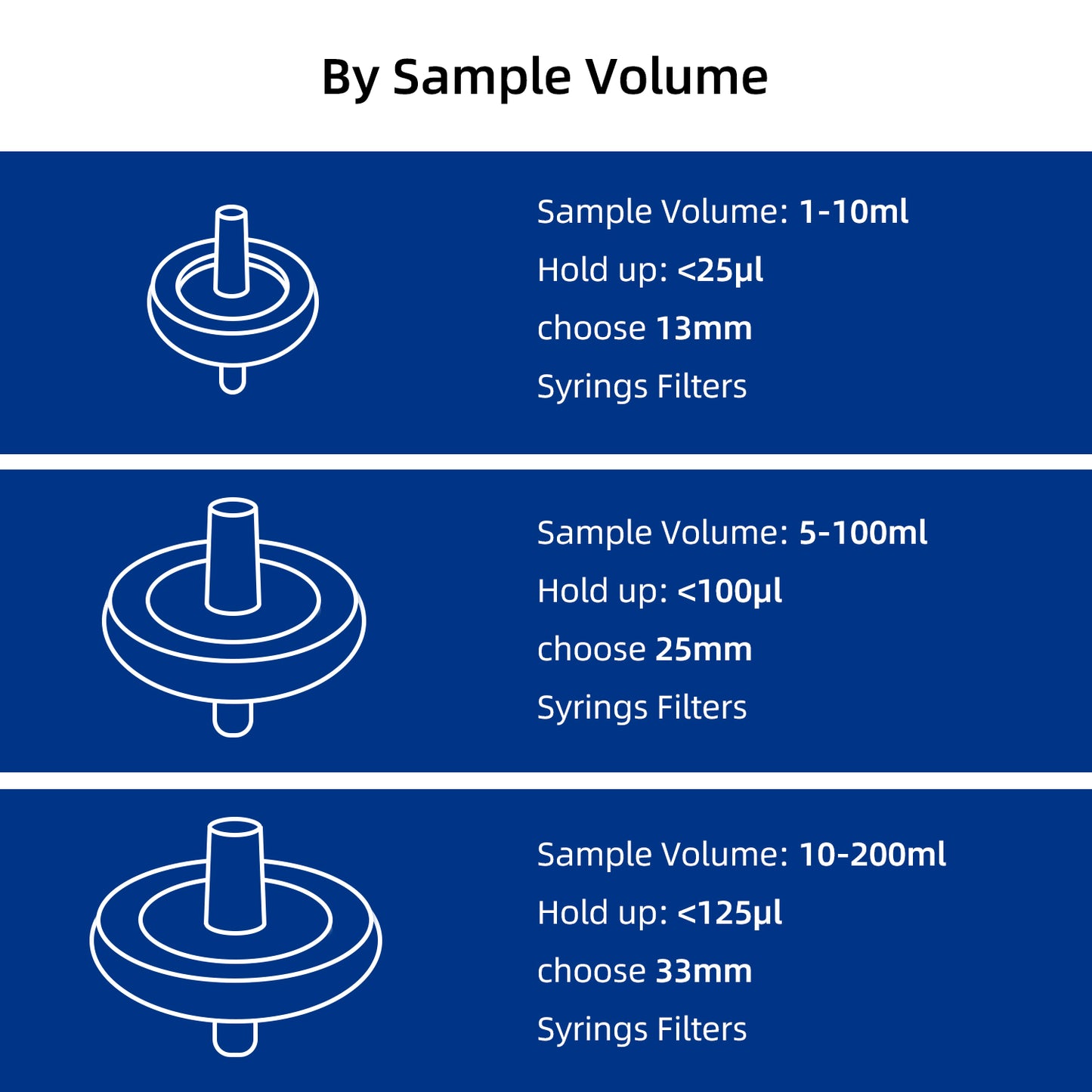
Select by Volume
| Sample Volume | Filter Diameter | Filtration Area | Hold-up Volume |
| < 10mL | 13mm | 0.72 cm² | < 25μl |
| 10mL to 100mL | 25mm | 3.4 cm² | < 100μl |
| 100mL to 200mL | 33mm | 4.5 cm² | < 125μl |
How to use a syringe filter?
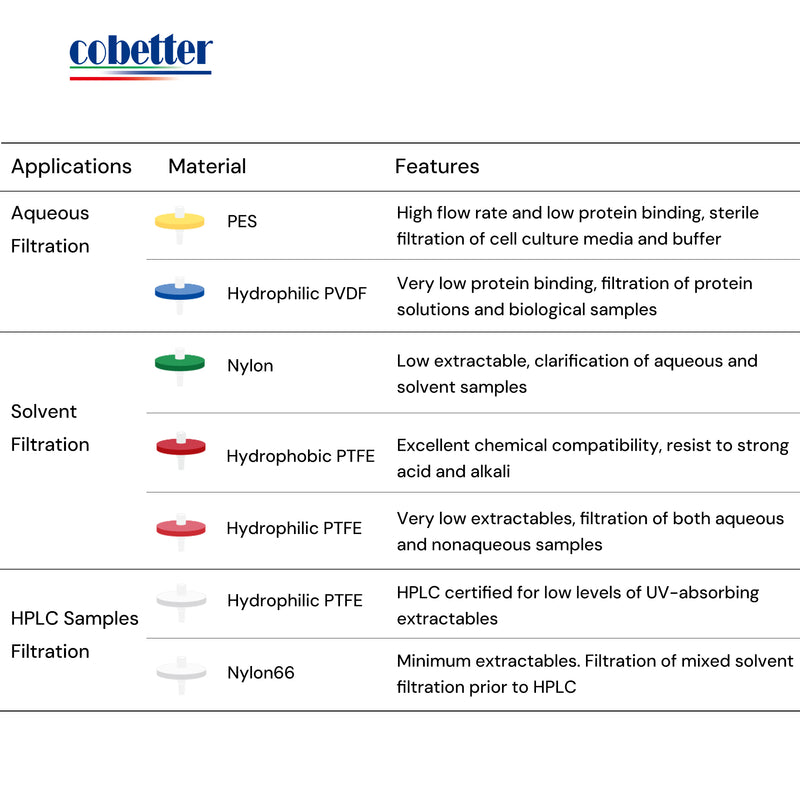
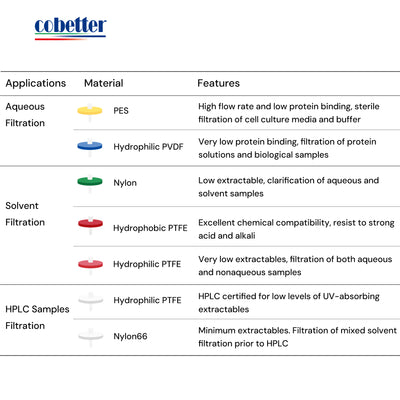
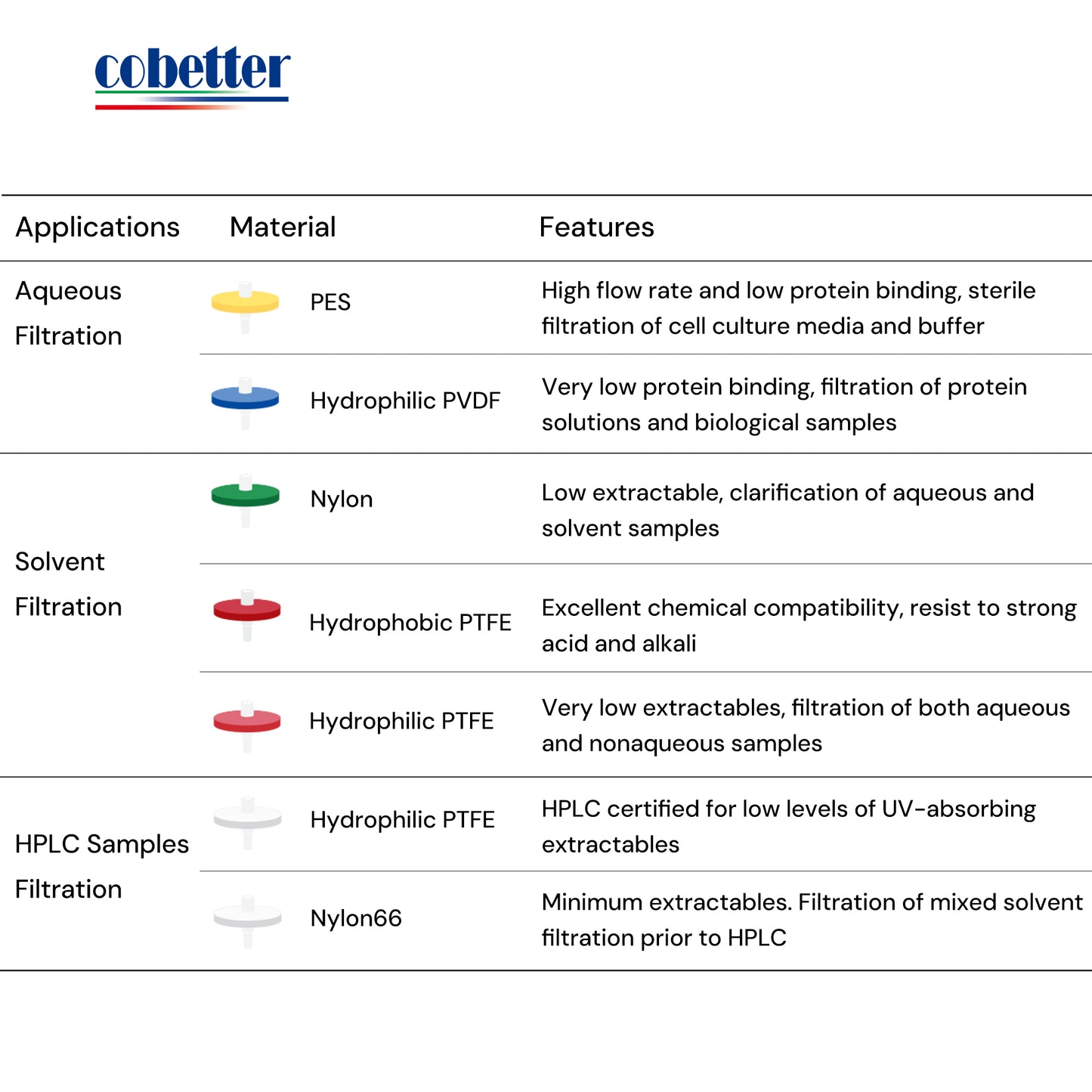
Choose the right type of membrane material based on the properties of your sample. Be careful of the chemical compatibility to avoid extractables and leachables. Read this article about How to choose a syringe filter.
1. Load the sample into the syringe.
2. Attach the filter to the luer-lock of the syringe with a twisting motion. Make sure it is securely attached.
3. Filter the sample. Hold the syringe and filter vertically, and gently press the plunger to push the sample through the filter. Take care not to apply too much force or you may damage the filter.
4. Discard the used filter and repeat for the next sample.
Sterilization Condition
Autoclaving
Only autoclavable syringe filters can be autoclaved. They can withstand autoclaving at 121°C (up to a maximum of 130°C) for 30 minutes, and this process can be repeated up to 20 times. However, we recommend using it only once for optimal performance after autoclaving. If customers wish to sterilize and reuse them, a thorough impact assessment is necessary.
Gamma Irradiation
Gamma irradiation involves exposing the filters to high-energy gamma rays, which can kill microorganisms such as bacteria, viruses and fungi. This method is typically used for large-scale sterilization in a relatively short time. Cobetter sterile syringe filters are gamma-irradiated and individually packed.